Understanding the periodic table is crucial for anyone interested in chemistry, as it unlocks the mysteries of matter and elements. Just as artists create digital cup artwork by mastering their medium, mastering the periodic table can empower learners to grasp essential scientific concepts.
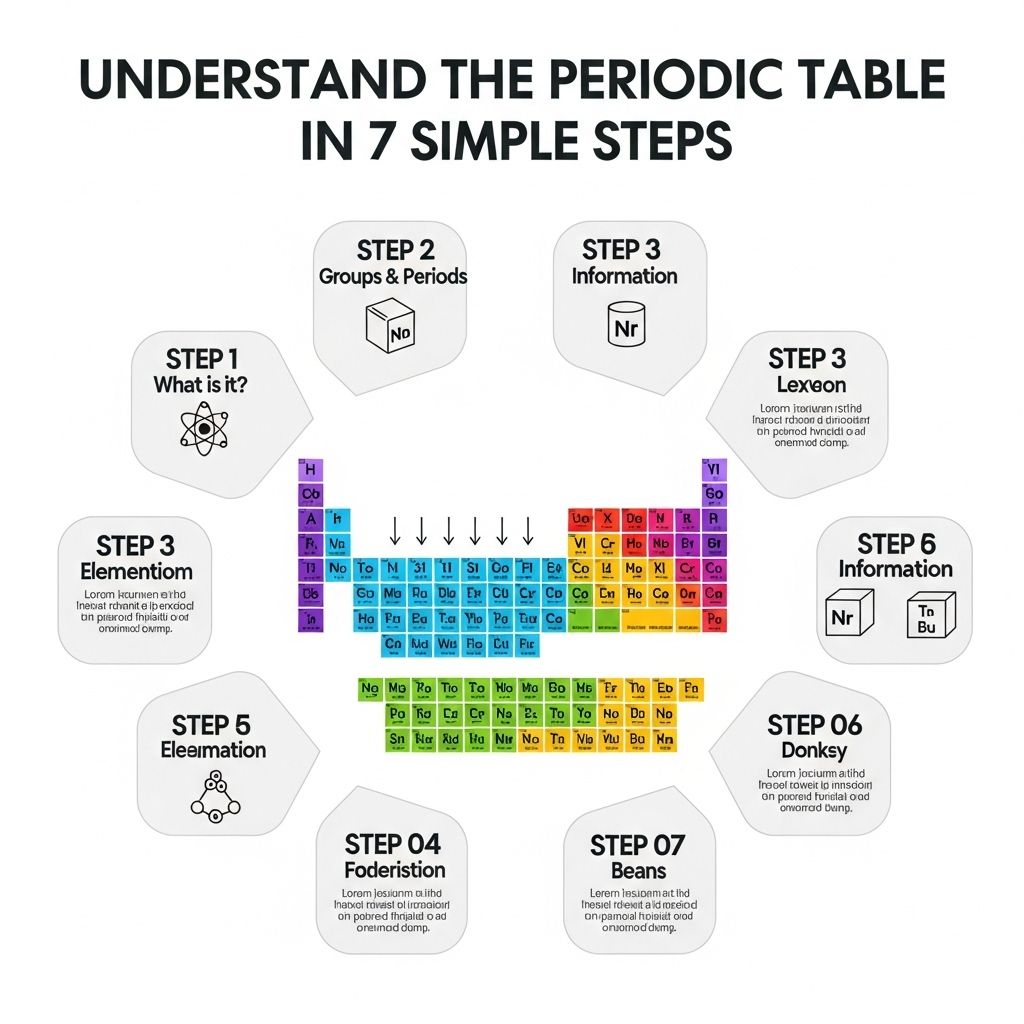
The periodic table is one of the most important tools in chemistry, offering a comprehensive overview of the elements that make up our world. Whether you’re a student, a teacher, or just a curious mind, understanding the periodic table can unlock the mysteries of matter. In this article, we will break down the periodic table into seven simple steps, making it easier to grasp its structure and significance.
Step 1: Know the Structure of the Periodic Table
The periodic table is organized in a grid format, with elements arranged by their atomic number, which is the number of protons in an atom’s nucleus. This organization reveals several important patterns and properties:
- Rows (Periods): Each row of the periodic table represents a period, with elements in the same period displaying similar energy levels.
- Columns (Groups or Families): Elements in the same column share common chemical properties, as they have the same number of valence electrons.
- Blocks: The periodic table is divided into blocks (s, p, d, f) based on the electron configuration of the elements.
Step 2: Understand Atomic Numbers and Mass
Each element is defined by its atomic number, which corresponds to the number of protons in its nucleus. Here’s a basic breakdown of these concepts:
| Element | Atomic Number | Atomic Mass |
|---|---|---|
| Hydrogen | 1 | 1.008 |
| Helium | 2 | 4.0026 |
| Lithium | 3 | 6.94 |
The atomic mass, located below the atomic number on the periodic table, is the average mass of an element’s isotopes.
Step 3: Explore Element Categories
The elements in the periodic table can be divided into several categories, which help to differentiate their properties:
1. Metals
Most of the elements are metals, typically shiny, conductive, and malleable. They are located on the left side and the center of the periodic table.
2. Nonmetals
Located on the right side, nonmetals are usually dull and poor conductors. They include gases like oxygen and nitrogen.
3. Metalloids
Found along the zig-zag line that divides metals and nonmetals, metalloids have properties of both. Examples include silicon and arsenic.
Step 4: Grasp the Significance of Groups
Understanding the groups of elements is crucial for predicting chemical behavior. Here are a few key groups:
1. Alkali Metals (Group 1)
These are highly reactive metals, such as sodium and potassium.
2. Alkaline Earth Metals (Group 2)
Less reactive than alkali metals, these include magnesium and calcium.
3. Halogens (Group 17)
These nonmetals, like chlorine and fluorine, are very reactive and often form salts with metals.
4. Noble Gases (Group 18)
Known for their lack of reactivity, these gases, including helium and neon, have a complete valence electron shell.
Step 5: Learn About Electron Configuration
Each element has a unique electron configuration that determines its chemical behavior. The arrangement of electrons around the nucleus can be summarized as follows:
- Electrons occupy energy levels (shells) around the nucleus.
- Each shell can hold a maximum number of electrons, defined by the formula 2n², where n is the shell level.
- Valence electrons, which are found in the outermost shell, play a vital role in chemical reactions.
Step 6: Familiarize Yourself with Trends
The periodic table reveals several trends that can be observed as you move across periods and down groups:
1. Atomic Radius
Generally increases down a group and decreases across a period due to increased nuclear charge.
2. Electronegativity
Tends to increase across a period and decrease down a group.
3. Ionization Energy
The energy required to remove an electron from an atom increases across a period and decreases down a group.
Step 7: Practical Applications of the Periodic Table
Understanding the periodic table has numerous applications in daily life and various fields:
- Chemistry and Material Science: Predicting chemical reactions and creating new materials.
- Medicine: Understanding the properties of elements and compounds used in pharmaceuticals.
- Environmental Science: Analyzing elements in pollutants and understanding chemical processes in nature.
In conclusion, the periodic table is more than just a collection of elements; it is a window into the fundamental nature of matter. By breaking it down into these seven steps, anyone can gain a greater appreciation and understanding of this crucial scientific tool. As you continue to explore the periodic table, remember that each element tells a story about the universe we inhabit.
FAQ
What is the periodic table and why is it important?
The periodic table is a systematic arrangement of chemical elements, organized by atomic number, electron configuration, and recurring chemical properties. It is crucial for understanding the relationships between different elements and predicting their behavior in chemical reactions.
How are elements arranged in the periodic table?
Elements are arranged in rows called periods and columns called groups. Elements in the same group share similar chemical properties, while elements in the same period show trends in properties as you move across the table.
What do the atomic number and atomic mass represent?
The atomic number represents the number of protons in an atom’s nucleus, which determines the element’s identity. The atomic mass is the weighted average mass of an element’s isotopes, reflecting both protons and neutrons.
What are metals, nonmetals, and metalloids on the periodic table?
Metals are typically shiny, malleable, and good conductors of heat and electricity. Nonmetals are usually dull, brittle, and poor conductors. Metalloids exhibit properties of both metals and nonmetals, making them versatile in various applications.
How can I use the periodic table to predict chemical reactions?
By understanding the position of elements in the periodic table, you can predict how they will react based on their group characteristics. For example, elements in Group 1 are highly reactive with water, while noble gases in Group 18 are largely inert.
What are some common trends in the periodic table?
Common trends include electronegativity, atomic radius, and ionization energy. Generally, electronegativity increases across a period and decreases down a group, while atomic radius decreases across a period and increases down a group.