In the world of chemistry, understanding acids and bases is essential for grasping various scientific concepts. Their roles extend beyond laboratories into everyday life, influencing cooking, cleaning, and industrial processes. For those looking to present their findings beautifully, exploring high-quality bag visuals can enhance the impact of your materials.
Understanding the concepts of acids and bases is fundamental in chemistry, and it serves as the cornerstone for various scientific principles and applications. From culinary uses to industrial processes, the roles of acids and bases are ubiquitous in our daily lives. This article aims to elucidate these concepts in a succinct manner, catering to those who want to grasp the essentials quickly.
The Basics of Acids and Bases
At the core of chemistry, acids and bases are defined based on their unique properties and behaviors in solutions. The most widely accepted theories, including the Arrhenius, Brønsted-Lowry, and Lewis definitions, provide insights into how we categorize these substances.
Arrhenius Definition
According to the Arrhenius definition:
- Acids release hydrogen ions (H+) in aqueous solutions.
- Bases release hydroxide ions (OH–) in aqueous solutions.
For example, hydrochloric acid (HCl) dissociates in water to produce H+, while sodium hydroxide (NaOH) dissociates to produce OH–.
Brønsted-Lowry Theory
The Brønsted-Lowry theory expands on the Arrhenius definition:
- Acids are proton donors.
- Bases are proton acceptors.
This theory explains the behavior of substances that do not fit the traditional definition, such as ammonia (NH3), which acts as a base by accepting a proton.
Lewis Theory
According to the Lewis theory, the definitions are further broadened:
- Acids are electron pair acceptors.
- Bases are electron pair donors.
This viewpoint allows for a more comprehensive understanding of acid-base reactions, particularly in non-aqueous solutions.
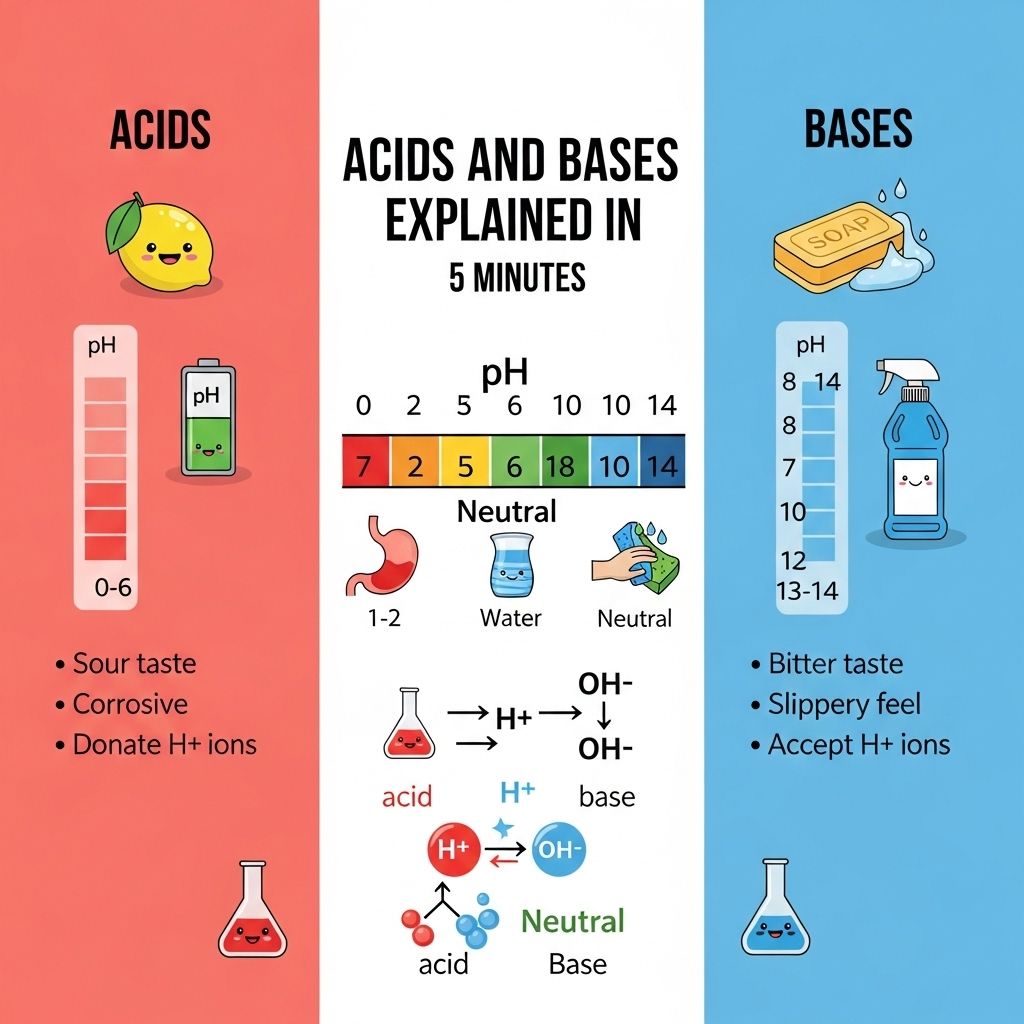
Properties of Acids and Bases
The distinct properties of acids and bases are easily identifiable and essential for various applications.
Common Properties of Acids
- Taste: Sour.
- pH: Less than 7.
- Indicators: Turn blue litmus paper red.
- Reactivity: React with metals to produce hydrogen gas.
- Conductivity: Conduct electricity in solution (due to ionization).
Common Properties of Bases
- Taste: Bitter.
- pH: Greater than 7.
- Indicators: Turn red litmus paper blue.
- Reactivity: Can react with acids to form salts and water.
- Texture: Often slippery.
The pH Scale
The pH scale is a crucial tool for measuring the acidity or basicity of a solution. It ranges from 0 to 14, with:
| pH | Nature |
|---|---|
| 0-6.9 | Acidic |
| 7 | Neutral |
| 7.1-14 | Basic |
A pH of 7 indicates a neutral solution, such as pure water, while solutions with pH less than 7 are classified as acids, and those greater than 7 are considered bases. The scale is logarithmic, meaning that each whole number change on the scale reflects a tenfold change in acidity or basicity.
Applications of Acids and Bases
Acids and bases play a vital role in numerous industries and everyday situations. Here are some notable applications:
1. Food and Cooking
Acids are commonly used in food preservation and flavoring, while bases are essential in baking.
- Vinegar (acetic acid): Used in pickling.
- Baking soda (sodium bicarbonate): Acts as a leavening agent.
2. Cleaning Agents
Many household cleaning products contain acids or bases to enhance cleaning efficacy.
- Acids: Citric acid in descaling products.
- Bases: Sodium hydroxide in drain cleaners.
3. Industrial Uses
In industries, acids and bases are utilized for a variety of chemical processes:
- Manufacture of fertilizers: Sulfuric acid is used to produce phosphoric acid.
- Metal processing: Hydrochloric acid is used for pickling metal to remove rust.
Neutralization Reactions
Neutralization reactions occur when an acid reacts with a base, resulting in the formation of water and a salt. This chemical reaction can be represented as:
Acid + Base → Salt + WaterFor example:
HCl + NaOH → NaCl + H2OIn this reaction, hydrochloric acid and sodium hydroxide yield sodium chloride (table salt) and water. Neutralization reactions are vital in various applications, from antacid tablets to environmental engineering.
Conclusion
Grasping the concepts of acids and bases equips individuals with a foundational understanding of chemistry. Whether in daily life or professional applications, recognizing their characteristics, behaviors, and interactions can lead to more informed decisions and a deeper appreciation of the chemical world around us. This concise overview serves as a stepping stone for further exploration into the fascinating realm of acids and bases.
FAQ
What are acids and bases?
Acids are substances that donate protons (H+) in a solution, while bases are substances that accept protons or donate hydroxide ions (OH-). They play crucial roles in chemical reactions and biological processes.
How do acids and bases differ in taste?
Acids typically have a sour taste, like lemon juice or vinegar, while bases usually taste bitter, such as baking soda. However, tasting chemicals is not recommended due to safety concerns.
What is the pH scale?
The pH scale ranges from 0 to 14 and measures the acidity or basicity of a solution. A pH less than 7 indicates an acidic solution, a pH of 7 is neutral, and a pH greater than 7 indicates a basic solution.
What are some common examples of acids and bases?
Common acids include hydrochloric acid (HCl) found in stomach acid and citric acid in citrus fruits. Common bases include sodium hydroxide (NaOH) found in drain cleaners and baking soda (sodium bicarbonate).
How do acids and bases react with each other?
When an acid reacts with a base, they undergo a neutralization reaction, producing water and a salt. This reaction helps maintain pH balance in various chemical and biological systems.
Why are acids and bases important in everyday life?
Acids and bases are essential in many everyday processes, including cooking, cleaning, and digestion. They are also crucial in industrial applications and environmental science.