Acids and bases are fundamental concepts in chemistry that influence various aspects of our daily lives, from cooking to environmental science. Grasping these concepts is crucial for anyone interested in the chemical processes that govern the world. For those looking to explore the interplay of design and science, mockup templates for bags can serve as a creative way to visualize ideas.
Understanding the fundamental concepts of acids and bases is essential not only for chemistry enthusiasts but also for anyone interested in the science that surrounds us every day. They play crucial roles in various fields, from biology and environmental science to industrial applications and cooking. This article delves into some of the key facts about acids and bases that everyone should know.
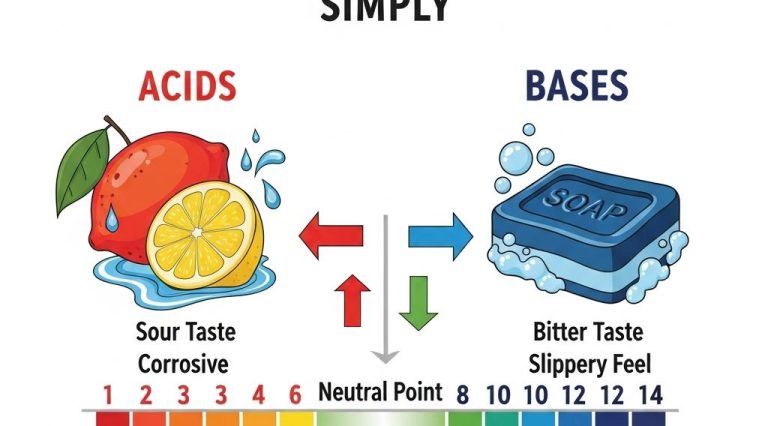
1. Definition of Acids and Bases
At the most basic level, acids and bases can be defined using the Brønsted-Lowry theory:
- Acids: Substances that donate protons (H+) in a chemical reaction.
- Bases: Substances that accept protons in a chemical reaction.
Another common definition is based on the pH scale, which measures how acidic or basic a solution is:
- A pH less than 7 indicates an acid.
- A pH of 7 indicates a neutral solution.
- A pH greater than 7 indicates a base.
2. The pH Scale and Its Importance
The pH scale ranges from 0 to 14 and is a logarithmic scale. This means that each whole number change on the scale represents a tenfold change in acidity or basicity:
| pH Level | Nature | Example |
|---|---|---|
| 0-3 | Strong Acids | Battery Acid (Sulfuric Acid) |
| 4-6 | Weak Acids | Vinegar (Acetic Acid) |
| 7 | Neutral | Pure Water |
| 8-10 | Weak Bases | Baking Soda (Sodium Bicarbonate) |
| 11-14 | Strong Bases | Drain Cleaner (Sodium Hydroxide) |
3. Common Household Acids and Bases
Many everyday household items contain acids or bases. Here are some common examples:
Acids
- Vinegar (Acetic Acid)
- Citrus Fruits (Citric Acid)
- Carbonated Drinks (Carbonic Acid)
- Stomach Acid (Hydrochloric Acid)
Bases
- Baking Soda (Sodium Bicarbonate)
- Soap (Fatty Acids and Bases)
- Ammonia (Ammonium Hydroxide)
- Bleach (Sodium Hypochlorite)
4. Acid-Base Reactions
When acids and bases interact, they undergo a chemical reaction known as neutralization:
Neutralization Reaction
The general reaction can be represented as:
Acid + Base → Salt + Water
For example:
HCl + NaOH → NaCl + H2O
In this reaction, hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to produce sodium chloride (table salt) and water.
5. The Role of Acids and Bases in Biology
Acids and bases are vital in biological systems. Enzymes, which facilitate biochemical reactions, often require specific pH levels to function effectively. For instance:
- The human stomach has a highly acidic environment (pH 1.5 to 3.5) that aids in digestion.
- Blood maintains a slightly basic pH (around 7.4) crucial for metabolic processes.
6. The Concept of Buffer Solutions
Buffer solutions are special mixtures that resist changes in pH when small amounts of acid or base are added. They play a critical role in maintaining the pH balance in biological systems and industrial processes. A common buffer system in the human body is:
- Bicarbonate Buffer System: HCO3–/H2CO3
7. Environmental Impact of Acids and Bases
Acids and bases significantly impact the environment. Acid rain, caused by sulfuric and nitric acids, can harm ecosystems and structures:
Effects of Acid Rain
- Damages forests and soil chemistry.
- Harms aquatic ecosystems by lowering pH.
- Corrodes buildings and monuments.
8. Industrial Applications
Acids and bases are extensively used in various industries. Here are some notable applications:
Acids
- Pharmaceuticals: Used in drug synthesis.
- Food Industry: Used for flavoring and preservation.
- Chemical Manufacturing: Important in producing fertilizers and plastics.
Bases
- Pulp and Paper Industry: Used in wood processing.
- Cleaning Products: Common in household cleaners.
- Textile Industry: Used in dyeing and finishing fabrics.
9. Acid-Base Indicators
Indicators are substances that change color based on the acidity or basicity of a solution. Some common acid-base indicators include:
- Litmus Paper: Turns red in acid and blue in base.
- Phenolphthalein: Colorless in acids; pink in basic solutions.
- Bromothymol Blue: Yellow in acid; blue in basic solutions.
10. Safety Considerations
Handling acids and bases requires caution. Here are important safety tips:
Safety Guidelines
- Always wear appropriate personal protective equipment (PPE) such as goggles and gloves.
- Work in a well-ventilated area to avoid inhaling fumes.
- Have neutralizing agents available in case of spills, such as sodium bicarbonate for acids.
In conclusion, acids and bases are essential components of chemistry and play significant roles in numerous fields. From medical applications to environmental impacts, understanding their properties and behaviors can lead to better decision-making both in everyday life and in scientific endeavors.
FAQ
What are acids and bases?
Acids are substances that donate protons (H+) in a solution, while bases are substances that accept protons or donate hydroxide ions (OH-).
What is the pH scale?
The pH scale ranges from 0 to 14 and measures the acidity or basicity of a solution, with 7 being neutral, below 7 acidic, and above 7 basic.
What are strong acids and bases?
Strong acids and bases completely dissociate in water, leading to a high concentration of H+ ions for acids and OH- ions for bases.
What are common examples of acids and bases?
Common examples of acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4), while common bases include sodium hydroxide (NaOH) and ammonia (NH3).
How do acids and bases react with each other?
When acids and bases react, they undergo a neutralization reaction, producing water and a salt.
Why are acids and bases important in everyday life?
Acids and bases play crucial roles in biological processes, industrial applications, and everyday products like cleaning agents and food items.